Abstract
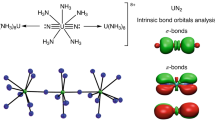
We report the isolation and spectroscopic identification of the eight-coordinated alkaline earth metal–dinitrogen complexes M(N2)8 (M=Ca, Sr, Ba) possessing cubic (Oh) symmetry in a low-temperature neon matrix. The analysis of the electronic structure reveals that the metal-N2 bonds are mainly due to [M(dπ)]→(N2)8 π backdonation, which explains the observed large red-shift in N-N stretching frequencies. The adducts M(N2)8 have a triplet (3A1g) electronic ground state and exhibit typical bonding features of transition metal complexes obeying the 18-electron rule. We also report the isolation and bonding analysis of the charged dinitrogen complexes [M(N2)8]+ (M=Ca, Sr).
Similar content being viewed by others
Introduction
The chemical conversion of dinitrogen to commercially useful commodities is one of the most important reactions in chemical industry, which has long been the subject of extensive research1. Activating the strong triple bond in N2 is a challenge for inventive chemists, who tried in the past various transition metals for binding the molecule as ligand in molecular complexes. Dinitrogen is a poorly binding donor due to its comparatively weak donor–acceptor interactions in transition metal complexes1,2. Metal–ligand bonds of N2 are usually much weaker than those of isoelectronic CO. Braunschweig and coworkers recently reported that boron compounds may also be utilized for fixation of dinitrogen3,4.
Very recently, we reported the isolation and spectroscopic identification of the eight-coordinated carbonyl complexes M(CO)8 of the alkaline earth atoms M=Ca, Sr, Ba possessing cubic (Oh) symmetry in a low-temperature matrix5. The analysis of the electronic structure showed that the classical main-group metals M bind the CO ligands via donor–acceptor interactions through their (n)d atomic orbitals thus mimicking transition metals. Now we found that the alkaline earth atoms may also bind N2 in octa-coordinated complexes M(N2)8 (M=Ca, Sr, Ba) whereby the loss of one N2 ligand has surprisingly only a slightly smaller bond dissociation energy than the dissociation of one carbonyl ligand from M(CO)8. Homoleptic dinitrogen complexes of genuine transition metals, such as tetra-coordinated Ni(N2)4, hexa-coordinated vanadium, and chromium complexes M(N2)6, have been synthesized and spectroscopically characterized in low-temperature matrices6,7,8,9. Mass spectrometric and infrared photodissociation spectroscopic investigations in the gas phase revealed that the Ti+, V+, and Nb+ cations form six-coordinated dinitrogen complexes10,11,12, while the Y+, La+, and Ce+ cations gave the octa-coordinated dinitrogen complexes13. There is only one theoretical report on neutral octa-coordinated dinitrogen complexes by Kovacs, who calculated the lanthanum species La(N2)n (n = 1–8)14. The finding that heavier alkaline earth atoms may bind eight N2 ligands in forming the neutral M(N2)8 complexes is unprecedented.
Results
Experimental studies
Figure 1 shows the spectra in the terminal N–N stretching frequency region from the experiment using a barium target and a 0.5% N2/Ne sample. The spectra were recorded after (a) 30 min of sample deposition at 4 K, (b) after annealing at 12 K, (c) after 15 min of visible light irradiation, and (d) after 15 min of UV-visible light irradiation. Only two bands at 2118.0 and 2237.6 cm−1 were observed. The 2237.6 cm−1 band is metal independent and can be assigned to the antisymmetric stretching vibration of the linear N4+ cation based on the literature data15. The 2118.0 cm−1 band increases tremendously under UV-visible light irradiation. The weak band at 2141 cm−1 is due to trace of CO impurity absorption.
Figure Full size table
The data in Table Full size table
The attractive metal–ligand interactions ΔEint of M(N2)8 come mainly from the covalent (orbital) term ΔEorb. The breakdown of the latter term into pairwise orbital interactions ΔEorb(1)–ΔEorb(5) shows that the covalent bonding is dominated by the [M(d)]→(N2)8 π backdonation ΔEorb(1), which contributes 70–85% to the total orbital interactions. The rather diffuse (n−1)d orbitals give the alkaline earth elements Ca–Ba an unusual reactivity with a remarkable donor ability. This was previously noted in our study of the carbonyl cation complexes [Ba(CO)]+ where the metal cation Ba+ is a donor for the neutral CO ligandFull size table